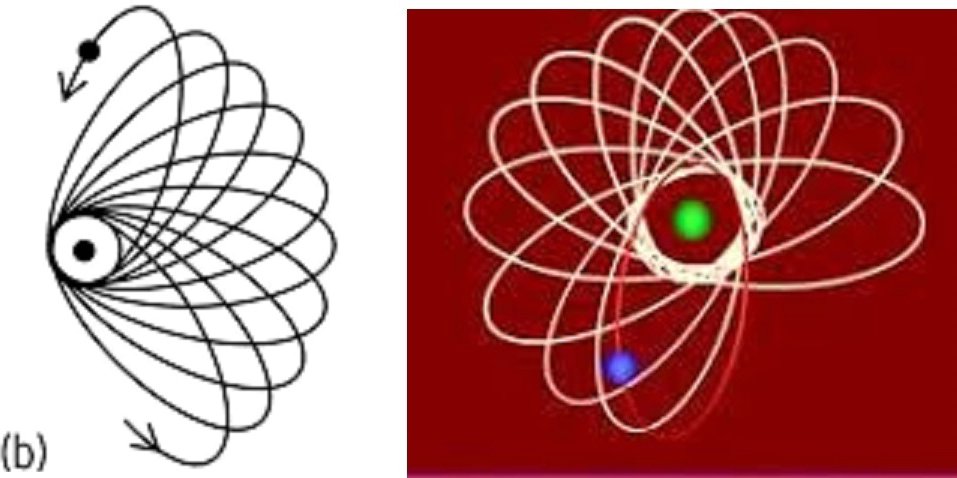
So, the picture shows the rotation of the elliptical orbit of an electron around the nucleus in the quantum mechanical model of the Bohr–Sommerfeld atom, which is INCORRECT from the point of view of modern quantum theory, because it “violates the principles” of Heisenberg’s “Uncertainty Relation” – one of the basic provisions of quantum mechanics. For such a model assumes complete certainty of both the momentum and the coordinates of the electron, which is precisely at odds with the “Ratio”, which states that it is impossible to determine both the momentum and the coordinate of a microparticle with any accuracy.
Electrons do NOT ROTATE in certain orbits around the nucleus, it follows from this principle, but are SPREAD out in a certain space with a certain probability around it.
If we accept this FUNDAMENTAL principle, which, in accordance with the religious dogmas of the Holy Scriptures of Physics, is MORE IMPORTANT THAN the experimental facts, then there are no stationary orbits in atoms in which, according to Bohr’s theory, electrons rotate, but there are only some vague clouds of the probability of electrons being somewhere nearby, right diagonally. Then a natural question arises:
Where do the free electrons in metals come from, flying in the crystal lattice at speeds of 600-2000 km/sec. If they are somehow “SMEARED” probabilistically, then what is flying in the metal? Free “probabilities”, free “uncertainty ratios”, or maybe free from any common sense psychopathic ideas of physicists that they have renamed into free electrons?
This is called the rich word “EUPHEMISM”, which means replacing a rude or obscene-sounding word with something more decent and normative. For example, instead of the rude word “WHORE,” a delicate set of words is used, such as “Priestess of love.” “Lady of the demimonde”, “Night Butterfly” – how beautiful and even romantic!?…
How do these “non-existent and indefinable exactly” have such PRECISE speeds?
Precisely because they fly at such speeds, and, not being strongly bound to the nucleus in metal atoms, being in outer orbits far from it, they fly off them at THEIR INHERENT SPEEDS!
Now the main thing is why this note was written!
Electrons in stationary orbits DO NOT RADIATE, contrary to the laws of electrodynamics. But WHAT happens to a rotating SOCKET if you look at it “from the side”, in the plane of the drawing?
FLUCTUATIONS IN THE DENSITY OF A NEGATIVE CHARGE, moving back and forth, oscillating with a high frequency and, therefore, EMITTING NON-QUANTUM electromagnetic waves in this plane.
This is true even for a hydrogen atom with only one electron in any orbit. The electron moves at a tremendous speed of a thousand kilometers per second, rotating in an orbit of a couple of Angstemes, which means that its “precession socket” of the elliptical orbit of the electron also rotates at high speed. For this “precession socket” itself, no one claimed anywhere that it was also STATIONARY!
It is an another mechanism for the appearance of phantom charges in any substance consisting of atoms.
And this again shows that the stability of atoms is not due to their “natural qualities of continuously non-emitting” particles, but to a certain dynamic equilibrium of energy loss and acquisition from the surrounding space.
Faciant melior potentes.
If I’m wrong, let my seniors correct me.
21 XI 2025